Facts about COVID-19
- The 2019 novel coronavirus disease (COVID-19) is a new virus of global health significance caused by infection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)
- COVID-19 is thought to spread from person to person in close contact through respiratory droplets
- These droplets can land in the mouths or noses of people who are nearby or possibly be inhaled into the lungs. It is also possible that a person can catch COVID-19 by touching a surface or object that has the virus on it and then touching their own mouth, nose, or possibly their eyes
Intended Use:
- The Vibrant COVID-19 Ab assay is a chemiluminescence immunoassay (CLIA) intended for the qualitative detection and differentiation of IgM and IgG antibodies to SARS-CoV-2 in human serum or Dry Blood Spot (DBS) using fingerstick blood specimen collected by the health care provider.
- The Vibrant COVID-19 Ab assay is intended for use as an aid in identifying individuals with an adaptive immune response to SARS-CoV-2, indicating recent or prior infection. At this time, it is unknown for how long antibodies persist following infection and if the presence of antibodies confers protective immunity.
- Results are for the detection of SARS CoV-2 antibodies. IgM and IgG antibodies to SARS-CoV-2 are generally detectable in blood several days after initial infection, although the duration of time antibodies present post-infection is not well characterized. Individuals may have detectable virus present for several weeks following seroconversion.
- Negative results do not preclude acute SARS-CoV-2 infection. If acute infection is suspected, direct testing for SARS-CoV-2 is necessary.
- False positive results for the Vibrant COVID-19 Ab assay may occur due to cross-reactivity from pre-existing antibodies or other possible causes.
- At this time, it is unknown for how long IgG antibodies may persist following infection.
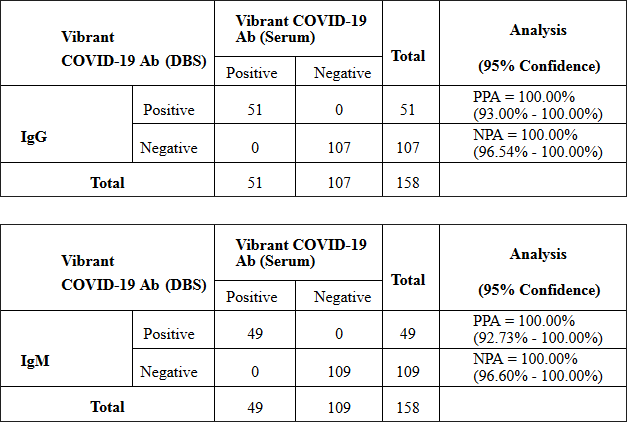
Dried Blood Spot studies
Individual IgG and IgM results for comparing serum and DBS:
Selection of card of DBS collection: Three different filter papers were evaluated for use as the DBS collection card. (Whatman 903 card, FTA DMPK-C card and Perkin Elmer 226). A panel of 50 DBS samples which includes 10 high positive samples, 25 samples with concentration ± 20% near cut-off, and 15 negative samples were tested for each card to evaluate the performance. Although the performance of all three cards were comparable, Whatman 903 card was selected for DBS collection due to better market availability.
DBS Drying time: The drying time was evaluated using 10 DBS cards for each time point from 30 min to 3 hours. Acceptance criteria was that the circular DBS spots are completely filled with blood saturating the area and no visible blood spot contamination on the flap of the DBS card. The spots are not wet after drying.
The drying time for Whatman 903 and Perkin Elmer 226 was 2 hours and for DMPK-C card was 3 hours at room temperature. The drying of the DBS card at room temp. for 2 hours before shipping was chosen for drying the collected DBS specimens on Whatman 903 card.
DBS Shipping Stability: A panel of 50 DBSsamples on Whatman 903 paper whichincludes 10 high positive samples, 25 samples with concentration ± 20% near cut-off, and 15negative samples were tested for stability study after cycling through the followingconditions (Table 13) as per International Safe Transit Association (ISTA) Procedure 7E (Testing Standard for Thermal Transport Packaging Used in Parcel Delivery System Shipment) and Procedure 7D (Temperature Test for Transport Packaging).
Table 13
| Temperature | Cycle Period | Cycle Period (hrs) | Total Time (hrs) |
| 40°C | 1 | 8 | 8 |
| 22°C | 2 | 4 | 12 |
| 40°C | 3 | 2 | 14 |
| 30°C | 4 | 36 | 50 |
| 40°C | 5 | 6 | 56 |
This study demonstrated that the antibody recovery from the DBS cards cycled through the above simulated shipping conditions was 91.2 – 96.2% for IgM and 91.4 – 99.2 for IgG for all five antigens. These data demonstrate that the DBS specimens can withstand the shipping conditions stress.
Please note:
- A patient cannot order their own tests
- A doctor’s requisition is required for all testing
Not Available in New York state
Only healthcare providers licensed in their state may order laboratory testing.
IgM and IgG Antibodies to:
- SARS-CoV-2 Nucleoprotein
- SARS-CoV-2 Spike Glycoprotein (S1)
- SARS-CoV-2 Spike Glycoprotein (S2)
- SARS-CoV-2 Receptor Binding Domain
Table 1: Overall Performance (IgM and IgG) of Vibrant COVID-19 Ab Assay
Vibrant COVID-19 Ab Assay
Clinical Diagnosis – NP Swab (RT-PCR) or Prior to Outbreak
Positive
Negative
Total
Analysis (95% Confidence)
Combined IgG/IgM
Positive
52
7
59
Positive Percent Agreement (PPA) = 98.11% (90.06% – 99.67%)
Negative
1
494
495
Negative Percent Agreement (NPA) = 98.60% (97.14% – 99.32%)
Total
53
501
554
Table 2 : The antibody results for the SARS-CoV-2 PCR positive specimens stratified based on the time in days between NP swab RT-PCR test results and the time serum was collected for the antibodies to SARS-CoV-2 antigens
| Days after NP swab RT-PCR test | # of Samples | Overall IgM + IgG | IgG PPA | IgM PPA |
|---|---|---|---|---|
| < 7 days | 7 | 85.71% | 71.43% | 85.71% |
| 7 – 14 days | 20 | 100% | 100% | 100% |
| > 14 days | 26 | 100% | 100% | 88.46% |
| total | 53 | 98.11% | 96.23% | 92.45% |
The CDC reports that the following symptoms may appear 2-28 days after exposure:
- Coughing
- Fever
- Shortness of breath
- Shortness of breath
- Gastrointestinal distress
-
- Diarrhea
- Abdominal cramping
- Fatigue
- Body aches
- Headaches
In order to place an order for the Vibrant COVID-19 test panel, you must have a provider account with Vibrant America. If you already have an account, please log in to your provider portal here to place orders:
If you do not have a provider account but wish to open one, free of charge, please fill out the form below to create an account so that you may begin ordering:
What type of test is your COVID-19 Panel?
The Vibrant COVID-19 test is a serological test. It requires 1 SST tube for specimen.
What do my test results mean?
At this time, Vibrant America is not able to provide clinical interpretation or consultation for interpretation or treatment guidance beyond the report comments on your lab results report. Please refer all questions to your ordering healthcare provider or a qualified and trained medical professional.
What is the sensitivity and the specificity of the Vibrant COVID-19 test?
The Vibrant COVID-19 test has a sensitivity of 98.1% and a specificity of 98.6%, with a coefficient of variation <5%.
Where does Vibrant America obtain lab kits and/or reagents for its COVID-19 test?
Vibrant America has its own technology platform which was tested and validated in the US population. Our preprint publication has more information on this. https://www.medrxiv.org/content/10.1101/2020.04.29.20085068v1.full.pdf+html We are FDA notified and pending EUA.
What are the control samples for Vibrant’s COVID-19 test? Are you using clinical samples as opposed to ‘spiked’ (i.e., artificial) samples?
Controls are clinically samples of individuals confirmed positive with NP swab test.
Are you using confirmed SARS-CoV-2 positive samples, and if so, how and when was this confirmed?
Antibody controls were from individuals who were confirmed positive using an NP swab test. These individuals tested swab positive in early March 2020.
If an antibody test, when was the sample collected in relation to a positive viral RNA test?
Serological samples were collected roughly an average >14 days from NP swab confirmation.
What are the demographics of the clinical test population?
This information is provided in our preprint publication under Table 1. https://www.medrxiv.org/content/10.1101/2020.04.29.20085068v1.full.pdf+html
Did the positive test population have symptoms in addition to a positive PCR test?
Yes. Our validation set included symptomatic and asymptomatic individuals.
Were pre-pandemic samples used in the negative test group?
Yes, samples from before the outbreak were used in the validation as a part of controls.
Did the assay evaluate samples that had exposure to other respiratory viruses such as influenza and RSV, as well as other coronaviruses, to determine cross reactivity and specificity?
Yes Table 1 from our preprint publication shows the complete set for cross reactivity controls. https://www.medrxiv.org/content/10.1101/2020.04.29.20085068v1.full.pdf+html
How many samples were used in the validation?
At the time of publication submission, we had more than 800 samples in the assay validation. Currently greater than 1500 samples have been used in the validation.
Are you doing progressive validation studies after the test is released?
Yes, we have a central IRB and more than 8 sites collecting samples for us.
- No At-home collection or At-home testing
- Vibrant COVID-19 Ab Assay should not be used to diagnose or exclude acute infection and should not be used as the sole basis for treatment or patient management decisions.
- It is unknown how long IgM or IgG antibodies to SARS-CoV-2 will remain present in the body after infection and if they confer immunity to infection.
Regulatory Statement
This test has not been FDA cleared or approved;
This test has been authorized by FDA under an EUA for use by authorized laboratories;
This test has been authorized only for the presence of antibodies against SARS-CoV-2, not for any other viruses or pathogens; and This test is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostic tests for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.